Loading ...
Loading ...
Loading ...
22
11. Reference to Standards
Device standard: Device corresponds to the requirements of the European standard for non-
invasive blood- pressure monitor
EN1060-1
EN1060-3
EN1060-4 – clinical investigation
IEC/EN 60601-1-11
ANSI / AAMI SP10, NIBP,
IEC80601-2-30:2009 + corrigendum 2010
Electrical compatibility: Device fulfils the stipulations of the
IEC/EN 60601-1 ,
IEC/EN 60601-1-2
The stipulations of the EU-Guidelines 93/42/EEC for Medical Products Class IIa have been fulfilled.
12. Remark:
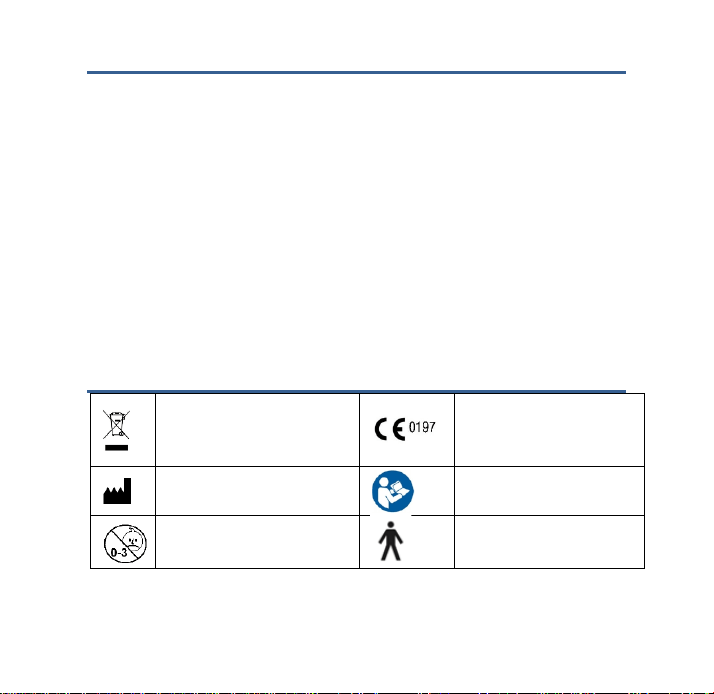
Some electrical and electrical
equipments forbid to abandon and
disposal at will
TUV NO.
Manufacturer’s name and address
Reading Instruction Book before
use
Inapplicable baby
Type B equipment
Loading ...
Loading ...
Loading ...