Loading ...
Loading ...
Loading ...
2_
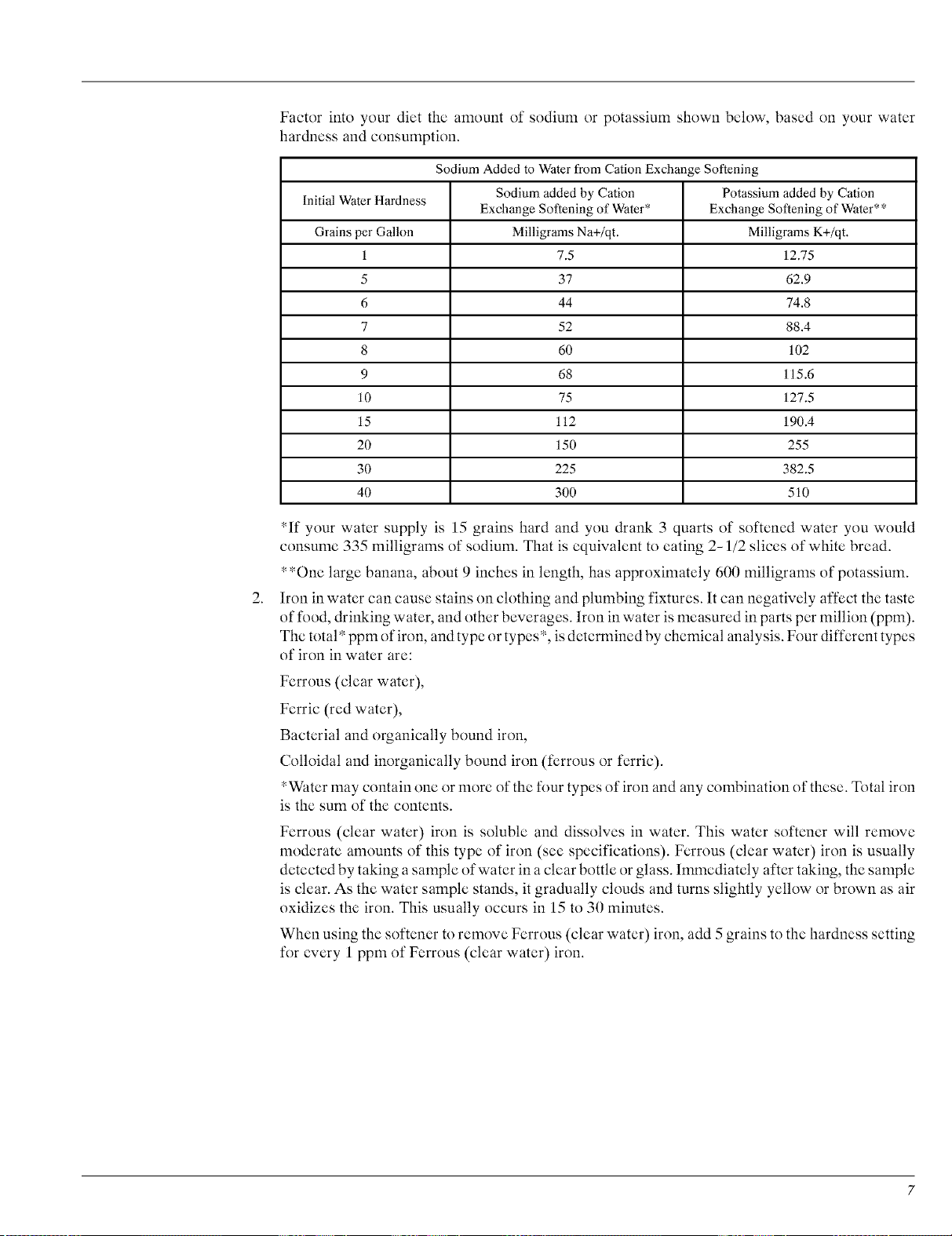
Factor into your diet the amount of sodium or potassium shown below, based on your water
hardness and consumption.
Sodium Added to Waterfrom CationExchange Softening
InitialWaterHardness Sodium addedby Cation Potassium added by Cation
Exchange Softeningof Water* Exchange Softeningof Water**
Grains perGallon MilligramsNa+/qt. MilligramsK+/qt.
1 7.5 12.75
5 37 62.9
6 44 74.8
7 52 88.4
8 60 102
9 68 115.6
10 75 127.5
15 112 190.4
20 150 255
30 225 382.5
40 300 510
*If your water supply is 15 grains hard and you drank 3 quarts of softened water you would
consume 335 milligrams of sodium. That is equivalent to eating 2-1/2 slices of white bread.
**One large banana, about 9 inches in length, has approximately 600 milligrams of potassium.
Iron in water can cause stains on clothing and plumbing fixtures. It can negatively affcct the taste
of t\_od, drinking water, and other beverages. Iron in water is measured in parts per million (ppm).
The total* ppm of iron, and type or types*, is determined by chemical analysis. Four different types
of iron in water are:
Ferrous (clear water),
Ferric (red water),
Bacterial and organically bound iron,
Colloidal and inorganically bound iron (fcrrous or ferric).
*Water may contain one or more of the four types of iron and any combination of these. Total iron
is the sum of the contents.
Ferrous (clear water) iron is soluble and dissolves in water. This water softener will remove
moderate amounts of this type of iron (see specifications). Ferrous (clear water) iron is usually
detected by taking a sample of water in a clear bottle or glass. Immediately after taking, the sample
is clear. As the water sample stands, it gradually clouds and turns slightly yellow or brown as air
oxidizes the iron. This usually occurs in 15 to 30 minutes.
When using the softener to remove Ferrous (clear water) iron, add 5 grains to the hardness setting
for every t ppm of Ferrous (clear water) iron.
Loading ...
Loading ...
Loading ...