Loading ...
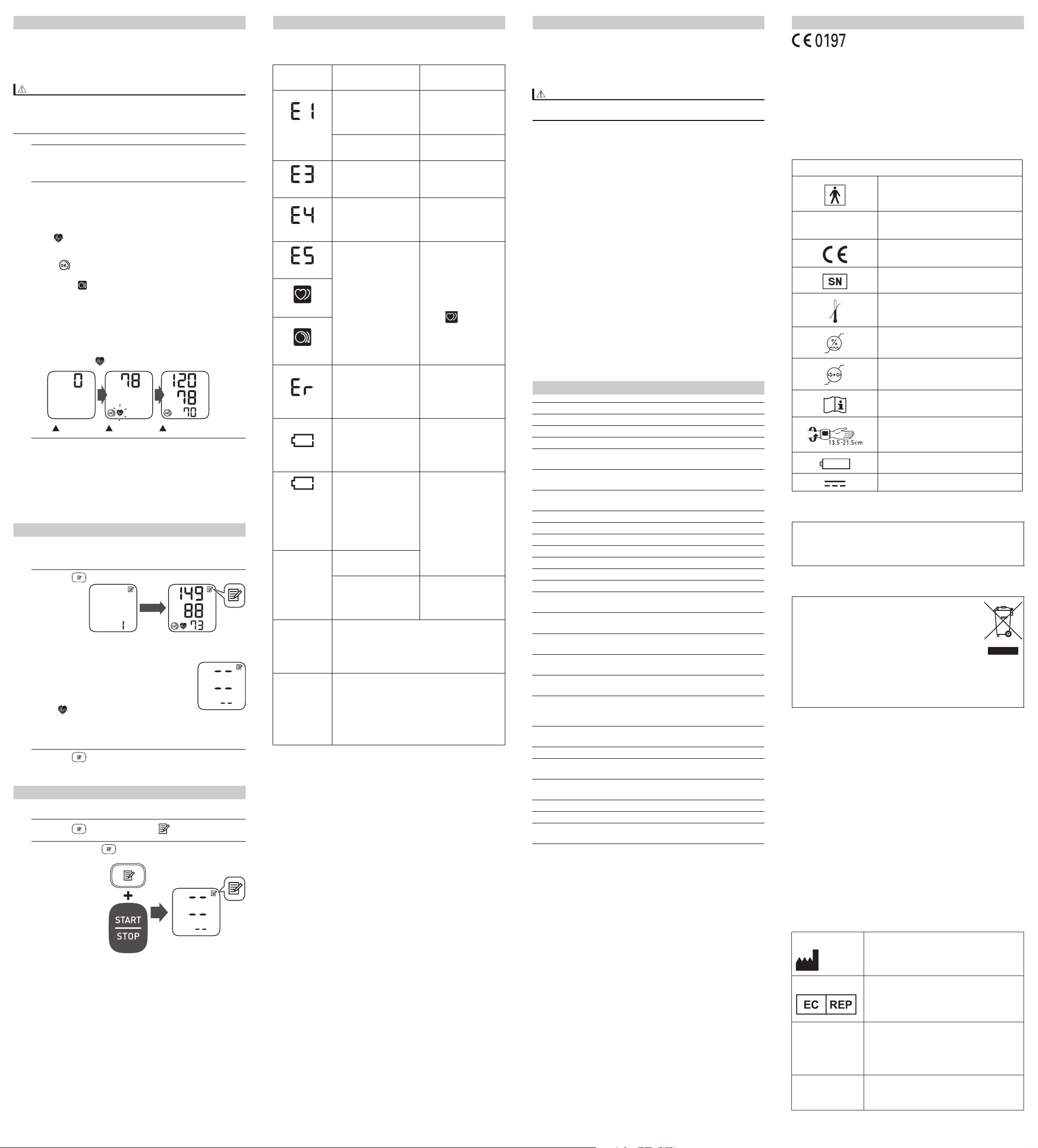
3.1 Taking a Measurement
Note
• To stop the measurement, press the [START/STOP] button once
to deflate the wrist cuff.
Caution
• DO NOT use this monitor with other medical electrical (ME)
equipment simultaneously. This may result in incorrect operation
of the monitor and/or cause an inaccurate reading.
• Remain still and DO NOT talk while taking a measurement.
1. Press the [START/STOP] button.
All symbols appear on the display before starting the
measurement.
2. Remain still and do not move or talk until the entire
measurement process is completed.
As the cuff inflates, your monitor automatically determines your
ideal inflation level. This monitor detects your blood pressure
and pulse rate during inflation.
The “ ” symbol flashes at every heartbeat.
Note
• The “ ” symbol appears if the wrist cuff is wrapped
around the wrist correctly.
• When the “ ” symbol is displayed, the wrist cuff is not
applied correctly. Press the [START/STOP] button to turn
your monitor off, then apply the cuff correctly.
After your monitor has detected your blood pressure and pulse
rate, the cuff automatically deflates. Your blood pressure and
pulse rate are displayed.
If either the systolic or the diastolic reading is high (refer to
section 1.3), the “ ” symbol appears.
3. Press the [START/STOP] button to turn your monitor off.
Note
• Your monitor will automatically turn off after 2 minutes.
• Wait 2-3 minutes between measurements. The wait time
allows the arteries to decompress and return to their pre-
measurement form. You may need to increase the wait time
depending on your individual physiological characteristics.
Your monitor automatically stores up to 30 readings.
4.1 Viewing the Readings Stored in Memory
1. Press the button.
The Memory number appears for one second before the pulse
rate is displayed. The most recent reading set is numbered “1”.
Note
• If there are no readings stored in the
memory, the screen to the right is displayed.
• If the reading is high (refer to section 1.3),
the “ ” symbol appears.
• If the memory is full, the monitor will delete the oldest
readings.
2. Press the button repeatedly to scroll through the
previous readings stored in the memory.
5.1 Deleting All Readings Stored in Memory
1. Press the button, then the “ ” symbol appears.
2. While holding the button down, press and hold the
[START/STOP] button for more than 2 seconds.
Note
• All readings will be deleted. You cannot partially delete the
readings stored in the memory.
3. Using Your Monitor
START
INFLATING COMPLETED
4. Using Memory Function
5. Other Settings
In case of any of the below problems occur during measurement,
first check that no other electrical device is within 30cm. If the
problem persists, please refer to the table below.
6. Error Messages and Troubleshooting
Display/
Problem
Possible Cause Solution
appears or the
wrist cuff does
not inflate.
The wrist cuff is not
applied correctly.
Apply the wrist cuff
correctly, then take
another measurement.
Refer to section 2.3.
Air is leaking from the
wrist cuff.
Contact local OMRON
representative.
appears
The wrist cuff is
overinflated exceeding
300 mmHg.
Do not touch the wrist
cuff while taking a
measurement.
appears
You move or talk during
a measurement.
Vibrations disrupt a
measurement.
Remain still and do not
talk during a
measurement.
appears
The pulse rate is not
detected correctly.
Apply the wrist cuff
correctly, then take
another measurement.
Refer to section 2.3.
Remain still and sit
correctly during a
measurement.
If the “ ” symbol
continues to appear, we
recommend you to
consult with your
physician.
appears
appears
appears
The monitor is
malfunctioned.
Press the [START/
STOP] button again. If
“Er” still appears,
contact local OMRON
representative.
flashes
Batteries are low.
Replacing all batteries
with 2 new alkaline
batteries is
recommended. Refer to
section 2.1.
appears or the
monitor is
turned off
unexpectedly
during a
measurement
Batteries are depleted.
Immediately replace all
batteries with 2 new
alkaline batteries. Refer
to section 2.1.
No power.
Nothing
appears on the
display of the
monitor.
Batteries are completely
depleted.
Battery polarities are not
properly aligned.
Check the battery
installation for proper
placement. Refer to
section 2.1.
Readings
appear too high
or too low.
Blood pressure varies constantly. Many factors
including stress, time of day, and/or how you
apply the wrist cuff, may affect your blood
pressure. Review sections 2.2 - 2.4 and chapter
3.
Any other
problems occur.
Press the [START/STOP] button to turn the
monitor off, then press it again to take a
measurement. If the problem continues, remove
all batteries and wait for 30 seconds. Then re-
install batteries.
If the problem still persists, contact local OMRON
representative.
7.1 Maintenance
To protect your monitor from damage, please follow the directions
below:
• Changes or modifications not approved by the manufacturer will
void the user warranty.
Caution
• DO NOT disassemble or attempt to repair this monitor or other
components. This may cause an inaccurate reading.
7.2 Storage
Keep your monitor in the storage case when not in use.
• Store your monitor in a clean, safe location.
Do not store your monitor:
• If your monitor is wet.
• In locations exposed to extreme temperatures, humidity, direct
sunlight, dust or corrosive vapors such as bleach.
• In locations exposed to vibrations or shocks.
7.3 Cleaning
• Do not use any abrasive or volatile cleaners.
• Use a soft dry cloth or a soft cloth moistened with neutral soap to
clean your monitor and wrist cuff, and then wipe them with a dry
cloth.
• Do not wash or immerse your monitor and wrist cuff in water.
• Do not use gasoline, thinners or similar solvents to clean your
monitor and wrist cuff.
7.4 Calibration and Service
• The accuracy of this blood pressure monitor has been carefully
tested and is designed for a long service life.
• It is generally recommended to have the unit inspected every two
years to ensure correct functioning and accuracy. Please consult
your authorised OMRON dealer or local OMRON representative at
the address given on the packaging or attached literature.
Note
• These specifications are subject to change without notice.
• In the clinical validation study, K5 was used on 85 subjects for
determination of diastolic blood pressure.
• This monitor is clinically investigated according to the
requirements of ISO 81060-2:2013.
• IP classification is degrees of protection provided by enclosures in
accordance with IEC 60529. This monitor is protected against
solid foreign objects of 12.5 mm diameter and greater such as a
finger, and against oblique falling water drops which may cause
issues during a normal operation.
• This device has not been validated for use on pregnant patients.
7. Maintenance
8. Specifications
Product description Wrist Blood Pressure Monitor
Model HEM-6161
Display LCD digital display
Cuff pressure range 0 to 299 mmHg
Blood pressure
measurement range
SYS: 60 to 260 mmHg
DIA: 40 to 215 mmHg
Pulse measurement
range
40 to 180 beats / min.
Accuracy
Pressure: ±3 mmHg
Pulse: ±5% of display reading
Inflation Automatic by electric pump
Deflation Automatic rapid deflation
Measurement method Oscillometric method
Operation mode Continuous operation
IP classification IP 22
Rating DC3 V 3.0 W
Power source 2 “AAA” alkaline batteries 1.5V
Battery life
Approximately 300 measurements (using
new alkaline batteries)
Durable period (Service
life)
5 years
Operating conditions
+10°C to +40°C / 15 to 90% RH (non-
condensing) / 800 to 1060 hPa
Storage / Transport
conditions
-20°C to +60°C / 10 to 90% RH (non-
condensing)
Weight
Approximately 85 g not including
batteries
Dimensions
Approximately 84 mm (w) × 62 mm (h) ×
21 mm (l)
(not including the wrist cuff)
Measurable wrist
circumference
13.5 to 21.5 cm
Memory Stores up to 30 readings
Contents
Monitor, storage case, 2 “AAA” alkaline
batteries, instruction manual
Protection against
electric shock
Internally powered ME equipment
Cuff Material Nylon and polyester
Applied part Type BF (wrist cuff)
Maximum temperature
of the applied part
Lower than +48°C
• This device fulfils the provisions of EC directive 93/42/EEC
(Medical Device Directive).
• This blood pressure monitor is designed according to the
European Standard EN1060, Non-invasive sphygmomanometers
Part 1: General Requirements and Part 3: Supplementary
requirements for electromechanical blood pressure measuring
systems.
• This OMRON product is produced under the strict quality system
of OMRON HEALTHCARE Co., Ltd., Japan. The Core component
for OMRON blood pressure monitors, which is the Pressure
Sensor, is produced in Japan.
Important information regarding Electro Magnetic Compatibility
(EMC)
Correct Disposal of This Product (Waste Electrical & Electronic
Equipment)
9. Guidance and Manufacturer’s Declaration
Symbols description
Applied part - Type BF
Degree of protection against electric
shock (leakage current)
Ingress protection degree provided by
IEC 60529
CE Marking
Serial number
Temperature limitation
Humidity limitation
Atmospheric pressure limitation
Need for the user to consult this
instruction manual.
Indicates the correct positioning for the
monitor on the wrist
Measurable wrist circumference
Battery
Direct current
HEM-6161 manufactured by OMRON HEALTHCARE Co., Ltd. conforms to
EN60601-1-2:2015 Electro Magnetic Compatibility (EMC) standard.
Further documentation in accordance with this EMC standard is available at
http://www.omronhealthcare-ap.com/emc-information. Refer to the EMC
information for HEM-6161 on the website.
This marking shown on the product or its literature, indicates that it
should not be disposed of, with other household wastes at the end
of its working life.
To prevent possible harm to the environment or human health from
uncontrolled waste disposal, please separate this product from
other types of wastes and recycle it responsibly to promote the
sustainable reuse of material resources.
Household users should contact either the retailer where they purchased this
product, or their local government office, for details of where and how they can
return this item for environmentally safe recycling.
Business users should contact their supplier and check the terms and conditions of
the purchase contract. This product should not be mixed with other commercial
waste for disposal.
IP XX
Made in Vietnam
Manufacturer
53, Kunotsubo, Terado-cho, Muko, KYOTO,
617-0002 JAPAN
EU-representative OMRON HEALTHCARE EUROPE B.V.
OMRON HEALTHCARE Co., Ltd.
Scorpius 33, 2132 LR Hoofddorp,
THE NETHERLANDS
www.omron-healthcare.com
www.omron-healthcare.com
Asia Pacific HQ OMRON HEALTHCARE SINGAPORE PTE
LTD.
438A Alexandra Road, #05-05/08
Alexandra Technopark, Singapore 119967
www.omronhealthcare-ap.com
Production
Facility
OMRON HEALTHCARE
MANUFACTURING VIETNAM CO., LTD.
Binh Duong Province, Vietnam