Loading ...
Loading ...
Loading ...
No lines seen
Blue control
line only
Pink/purple
sample line only
Blue control line AND
pink/purple sample line
No lines seen
Blue control
line only
Pink/purple
sample line only
Blue control line AND
pink/purple sample line
No lines seen
Blue control
line only
Pink/purple
sample line only
Blue control line AND
pink/purple sample line
B. Check for Negative COVID-19 Result
If you see any of these, the test is invalid. An invalid result means this
test was unable to determine whether you have COVID-19 or not. A
new test is needed to get a valid result.
Please contact Technical Support at + 1 833-637-1594
Throw away all used
test kit components in
the trash.
C. Check for Invalid Result
CONTROL
SAMPLE
No lines seen
Blue control
line only
Pink/purple
sample line
only
Blue control line
AND pink/purple
sample line
No lines seen
Blue control
line only
Pink/purple
sample line only
Blue control line AND
pink/purple sample line
Please share your test result with your health care provider.
If used for serial testing, a second test should be obtained over three
days with at least 36 hours between tests.
DISPOSE THE TEST KIT
E.
DO NOT remove swab.!
Keep card FLAT on table.!
3x
Note: False negative result can occur if swab is not turned.
Find result window and look carefully for two pink/purple lines.
Positive Result: If you see two pink/purple lines (one on the top half
and one on the bottom half), this means COVID-19 was detected.
Below are photos of actual positive tests. On the right, note how faint
the bottom line can get.
Look very closely!
The bottom line
can be very faint.
Any pink/purple
line visible here is
a Positive Result.
!
DO NOT move or touch the card during this time.
9. Wait 15 minutes.
Read the result at 15
minutes.
Do not read the result
before 15 minutes or after
30 minutes.
Find result window and look for a single pink/purple line in window.
Negative Result: If you see only one pink/purple line on the top half,
where it says “Control
„
this means COVID-19 was not detected.
CONTROL
SAMPLE
No Line
Negative
No Line
Negative
REPORT YOUR RESULTS
F.
BinaxNOW
™
COVID-19 Ag
CARD
SAMPLE
CONTROL
BinaxNOW
™
COVID-19 Ag
CARD
SAMPLE
CONTROL
BinaxNOW
™
COVID-19 Ag
CARD
SAMPLE
CONTROL
7. Turn swab to right 3 times
to mix the swab with
the drops.
Do not skip this step.
Leave the swab in the card
for the remainder of the test.
A
B
8. Peel adhesive liner o. Be
careful not to touch other
parts of card.
Close left side of card over
swab. Press firmly on the
two lines on right edge of
card to seal.
Keep card face up on table.
WAIT
15 MINUTES
READ
AT 15-30 MINUTES
15
min
Note: A control line may appear in the result window in a few
minutes but a sample line may take as long as 15 minutes to appear.
Note: Results should not be read after 30 minutes.
Note: See other side to read about what your results mean.
6. Insert swab tip into
bottom hole.
Firmly push the swab tip
from the bottom hole until
it is visible in the top hole.
Do not remove the swab
from the card.
PERFORM THE TEST
C.
INTERPRET RESULTS
D.
A. Check for Positive COVID-19 Result
CONTROL
SAMPLE
PositivePositive
Solid Line Faint Line
OR
Solid Line
Positive
Faint Line
Positive
Peel
Seal
RESULT INTERPRETATION
Positive Result
A positive test result for COVID-19 indicates that antigens from SARS-CoV-2 were detected, and
the patient is very likely to be infected with the virus and presumed to be contagious. Test results
should always be considered in the context of clinical observations and epidemiological data (such
as local prevalence rates and current outbreak/epicenter locations) in making a final diagnosis and
patient management decisions. Patient management should follow current CDC guidelines.
Negative Result
A negative test result for this test means that antigens from SARS-CoV-2 were not present in the
specimen above the limit of detection. However, a negative result does not rule out COVID-19
and should not be used as the sole basis for treatment or patient management decisions, including
infection control decisions. The amount of antigen in a sample may decrease as the duration of
illness increases. Negative results should be treated as presumptive and confirmed with a molecular
assay, if necessary, for patient management.
For serial testing programs, additional confirmatory testing with a molecular test for negative results
may be necessary, if there is a high likelihood of COVID-19, such as, an individual with a close
contact with COVID-19 or with suspected exposure to COVID-19 or in communities with high
prevalence of infection. Additional confirmatory testing with a molecular test for positive results
may also be necessary, if there is a low likelihood of COVID-19, such as in individuals without
known exposures to COVID-19 or residing in communities with low prevalence of infection.
LIMITATIONS
• This test detects both viable (live) and non-viable, SARS-CoV, and SARS-CoV-2. Test
performance depends on the amount of virus (antigen) in the sample and may or may not
correlate with viral culture results performed on the same sample.
• A negative test result may occur if the level of antigen in a sample is below the detection limit of
the test.
• The performance of the BinaxNOW COVID-19 Antigen Self Test was evaluated using the
procedures provided in this product insert only. Modifications to these procedures may alter the
performance of the test.
• False negative results may occur if a specimen is improperly collected or handled.
• False negative results may occur if inadequate extraction buer is used (e.g., <6 drops).
• False negative results may occur if specimen swabs are not twirled within the test card.
• False negative results may occur if swabs are stored in their paper sheath after specimen
collection.
• Positive test results do not rule out co-infections with other pathogens.
• False negative results are more likely after eight days or more of symptoms.
• Positive test results do not dierentiate between SARS-CoV and SARS-CoV-2.
• Negative test results are not intended to rule in other non-SARS viral or bacterial infections.
• The presence of mupirocin may interfere with the BinaxNOW COVID-19 Antigen Self Test
and may cause false negative results.
• Negative results are presumptive, do not rule out COVID-19 infection and it may be necessary
to obtain additional testing with a molecular assay, if needed for patient management.
• Performance of nasal swabs collected from patients without symptoms or other epidemiological
reasons to suspect COVID-19 infection or for serial screening, when tested twice over three
days with at least 36 hours between tests has not been determined, a study to support use will
be completed.
• If the dierentiation of specific SARS viruses and strains is needed, additional testing, in
consultation with state or local public health departments, is required.
• The performance of this test was established based on the evaluation of a limited number
of clinical specimens collected in November 2020. The clinical performance has not been
established in all circulating variants but is anticipated to be reflective of the prevalent variants in
circulation at the time and location of the clinical evaluation. Performance at the time of testing
may vary depending on the variants circulating, including newly emerging strains of SARS-
CoV-2 and their prevalence, which change over time.
PERFORMANCE CHARACTERISTICS
Clinical Performance
Clinical performance characteristics of BinaxNOW COVID-19 Antigen Self Test was evaluated
in an ongoing multi-site prospective study in the U.S. A total of four (4) investigational sites
throughout the U.S. participated in the study. To be enrolled in the study, patients had to
be presenting at the participating study centers with suspected COVID-19 within 7 days of
symptom onset. Each Subject was provided a BinaxNOW COVID-19 Antigen Self Test. Under
the observation and coaching of a clinical site sta member trained as a proctor, the Subject self-
collected one (1) nasal swab and performed the BinaxNOW COVID-19 Antigen Self Test. Test
results were interpreted and recorded by the Subject or other home user and independently by the
proctor. Parents of pediatric Subjects under the age of 14 or Legally Authorized Representatives
of adult Subjects unable to perform self-collection collected one (1) nasal swab from the Subject,
performed the BinaxNOW COVID-19 Antigen Self Test, then interpreted and recorded the result
for the patient.
An FDA Emergency Use Authorized real-time Polymerase Chain Reaction (RT-PCR) assay for
the detection of SARS-CoV-2 was utilized as the comparator method for this study.
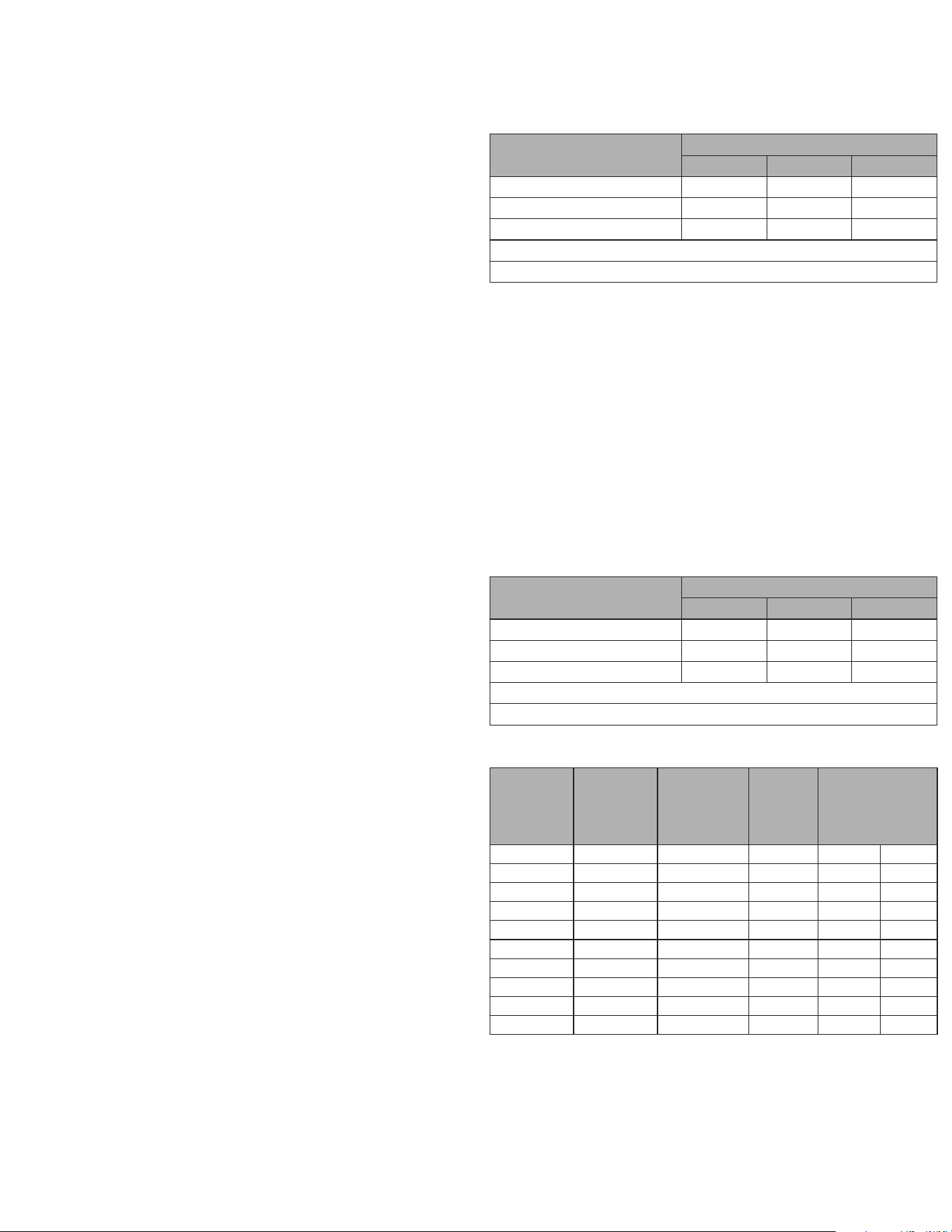
The performance of BinaxNOW COVID-19 Antigen Self Test was established with 53 nasal swabs
collected from individual symptomatic patients (within 7 days of onset) who were suspected of
COVID-19.
BinaxNOW
™
COVID-19 Antigen Self Test Performance within 7 days of symptom onset against
the Comparator Method
BinaxNOW
™
COVID-19 Ag 2 Card
Home Test
Comparator Method
Positive Negative Total
Positive 22 0 22
Negative 2 28 30
Total 24 28 52*
Positive Agreement: 22/24 91.7% (95% CI: 73.0% - 98.9%)
Negative Agreement: 28/28 100.0% (95% CI: 87.7% - 100.0%)
*1 sample generated an invalid BinaxNOW COVID-19 Ag 2 Card result (0.1% invalid rate)
The performance of this test has not yet been clinically validated for use in patients without signs
and symptoms of respiratory infection or for serial screening applications, and performance may
dier in these populations.
Performance of BinaxNOW COVID-19 Antigen Self Test, with the test performed and results
interpreted by the home user is similar to performance obtained by test operators with no
laboratory experience. Due to the relatively small sample size for the home use clinical study, at
the time of the interim analysis, the BinaxNOW COVID-19 Antigen Self Test positive agreement
established in this ongoing clinical study is estimated to be between 73.0% and 98.9% as reflected
in the 95% Confidence Interval. This is consistent with the performance established in a separate
multi-site study in the US, where the BinaxNOW COVID-19 Ag Card test was performed and
results interpreted by test operators with no laboratory experience. In that study, BinaxNOW
COVID-19 Ag Card test positive agreement was 84.6% (95% CI: 76.8% - 90.6%), refer below:
The performance of BinaxNOW COVID-19 Ag Card was established with 460 nasal swabs
collected from individual symptomatic patients (within 7 days of onset) who were suspected of
COVID-19.
BinaxNOW COVID-19 Ag Card Performance within 7 days of symptom onset against the
Comparator Method
BinaxNOW COVID-19 AgCard
Comparator Method
Positive Negative Total
Positive 99 5 104
Negative 18 338 356
Total 117 343 460
Positive Agreement: 99/117 84.6% (95% CI: 76.8% - 90.6%)
Negative Agreement: 338/343 98.5% (95% CI: 96.6% - 99.5%)
Patient demographics, time elapsed since onset of symptoms for all patients enrolled in the above
study, are presented in the table below. Positive results broken down by days since symptom onset:
Days Since
Symptom
Onset
Cumulative
RT-PCR
Positive (+)
Cumulative
BinaxNOW
COVID-19
Antigen Self
Test Positive (+)
PPA
95 % Confidence
Interval
1 12 10 83.3% 51.6% 97.9%
2 34 28 82.4% 65.5% 93.2%
3 50 41 82.0% 68.6% 91.4%
4 63 50 79.4% 67.3% 88.5%
5 78 63 80.8% 70.3% 88.8%
6 90 75 83.3% 74.0% 90.4%
7 117 99 84.6% 76.8% 90.6%
8 to 10 144 118 81.9% 74.7% 87.9%
11 to 14 161 126 78.3% 71.1% 84.4%
All specimens 167 129 77.2% 70.1% 83.4%
A cohort of patients who presented with symptom onset greater than seven days were enrolled in
the clinical study (n = 161). The positive agreement in patients with symptoms greater than seven
days was 60% (30/50) and negative agreement was 98% (109/111). Therefore, negative results in
patients with symptom onset greater than seven days should be interpreted with caution, as the
sensitivity of the assay decreases over time.
Loading ...
Loading ...
